
Danielle Darnault and her daughter Lila, who is 7, were often frustrated that others didn’t take Lila’s peanut allergy seriously. Lila participated in the clinical trial at UCLA and can now tolerate enough peanut to allow her to sit with her friends during school lunch. PHOTO COURTESY DANIELLE DARNAULT
One afternoon about five years ago, Danielle Darnault was shopping at a Target store in Culver City with her 1-year-old daughter Lila. Lila got hungry, so Darnault opened a box of granola bars she was tossing into her cart and handed one to her toddler. “Just holding the granola bar, her hand got really red,” Darnault says. “She put it to her mouth and her whole face blew up in hives, and she started screaming and crying.”
Darnault immediately texted her pediatrician, who advised her to give Lila Benadryl right there in the store, then drive straight to the hospital. In the car, Lila’s eyes began to swell shut and she screamed and cried the entire way. In the emergency department of Ronald Reagan UCLA Medical Center, doctors gave Lila a drug called epinephrine and sent Darnault and Lila home with an EpiPen and a warning: Do not let Lila eat foods that contain or might contain peanuts, or that were made in facilities or kitchens where peanuts are used. Her peanut allergy is a matter of life and death.
Lila’s story is not new, but scientists are working to change the story for her and more than 1.6 million kids and teens like her in the U.S. who have peanut allergy. And they think they might have found the key.
When Lila’s body came into contact with the peanuts in that granola bar, her immune system saw the peanut as an invader – as if it were an infectious bacteria or virus – and flooded her body with chemicals designed to fight off the threat. But with nothing to fight, the chemicals sent her body into shock instead. This is called an anaphylactic reaction, and a typical one begins like Lila’s did with redness, itching and hives. As the reaction progresses, the blood pressure drops and the airways, throat and tongue can swell, making breathing extremely difficult. If the reaction isn’t stopped with epinephrine, breathing and heartbeat can stop and the person can die.
A Tough Trial
People with allergies to things in the environment – pollen or animal dander, for instance – have long been able to seek some relief through allergen immunotherapy, where doctors inject tiny, carefully controlled amounts of the allergen into the body to build up a tolerance. No such option has existed for those with food allergies because their chance of having a life-threatening reaction to the therapy is much higher – 90 percent, compared with just 1 percent for those with environmental allergies.
But in recent years, with advances in the field, researchers have been testing a therapy that could prove a game changer for those allergic to peanuts – chosen for this research because it is the deadliest of the food allergens. And in an article published in November in the New England Journal of Medicine, they described the largest study of this therapy, called oral immunotherapy or OIT, to date.
Conducting such a study, in this case a phase-three clinical trial of a drug called AR101, isn’t simple, as allergist/immunologist Rita Kachru of UCLA Medical Center, a principal investigator in the study, explains. UCLA was one of 35 study centers participating in the trial and enrolled nine patients. To find those nine, they meticulously tested patients’ reactions to peanuts, and enrolled those who had allergic responses to less than 100 mg, the equivalent of about one-third of a peanut.
The trial was double-blind, meaning that one group of patients received AR101, another group received a placebo, and neither the patients nor the researchers knew which patients were which.
Patients on the therapy started by receiving a 1 mg dose of AR101, which is essentially carefully measured peanut flour, daily. The goal was to safely work their way up to a dose the equivalent of one full peanut, which is about 300 mg. It took six months of weekly increases to build up to that dose. Participants stayed on a 300-mg dose for six months, then had a food challenge, eating increasing amounts of peanut until they reacted. And the results were promising. Two-thirds of the patients on AR101, none of whom had been able to tolerate one-third of a peanut without an allergic reaction, could now tolerate two whole peanuts.
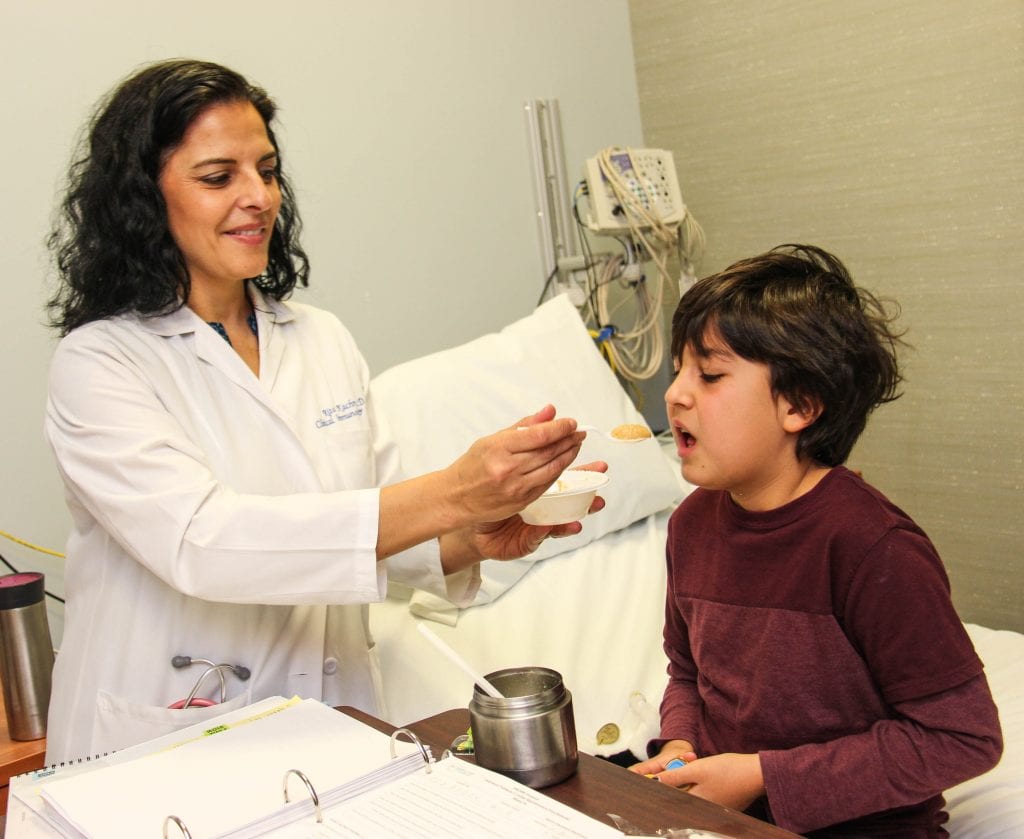
Allergist/immunologist Rita Kachru, M.D., has a desensitization session with patient Wyeth Holliss Sander, 10, as part of a clinical trial of oral immunotherapy for peanut allergy at UCLA Health. Before his two years of therapy, Sander couldn’t risk eating in a frozen yogurt shop because of possible contact with peanut. Now he can tolerate up to six peanuts without allergic reaction. PHOTO BY ROBERT HERNANDEZ/UCLA
The therapy didn’t work for everyone. “This was a really difficult trial for our patients,” says Kachru. Around 20 percent of patients who started had to leave the trial early, many because of gastrointestinal side effects caused by the medication, including upset stomach and uncontrolled reflux. And a few patients (less than 2 percent) did experience anaphylactic reactions during the trial. Some patients reacted at the clinic when they moved up to the next-largest dose of AR101. Lila Darnault, who is now 7, had a serious reaction at home because, for a few days, a nanny hadn’t been making sure she took her full dose of the medication. The next day, when her mom did give the full dose, they had to call an ambulance.
Ready to Rescue
Why go to the trouble, then, for something that seems like not that big a deal?
Because peanut allergy can be terrifying and can hamper a child’s quality of life. “The patients who were interested in doing the study were patients who needed to do something like this because their peanut allergy was affecting their lifestyle,” Kachru says. “Either they were afraid to go out to restaurants, they were afraid to fly or they were having recurring reactions.”
And managing a nut allergy at the level that these kids have isn’t simple at all. Label-reading is a good start, but because there is a risk with even trace amounts of peanut, it isn’t enough. Families always need to be prepared for an allergy emergency.
The Darnault family handles this by taking their “rescue pack” with them wherever they go. It includes an EpiPen Jr. twin pack (because sometimes you have to use two), a drug called famotidine for GI symptoms, the immune-suppressing drug prednisone, the antihistamine Benadryl and the long-acting allergy medication Zyrtec. “We also, with that, keep lollipops,” Darnault says. “In the event this happens, you need something to cheer you up.”
Another issue is that these allergies often aren’t taken seriously. Darnault visited the West L.A. charter school where Lila, who is now in second grade, was starting kindergarten six months before the beginning of the school year to talk about Lila’s allergy. But when school began, the campus still had no plan for keeping Lila safe at lunchtime.
At Back To School night, Lila’s kindergarten teacher announced that there was a child with a life-threatening nut allergy in the class and asked families not to send peanut products to school with their children. “The dad sitting next to me said to another dad, ‘This is BS. I can pack whatever I want for my kid,’” Darnault says. When she approached the dad and tried to explain the seriousness of the situation, his response was, “Well, that just doesn’t make any sense. Your kid’s not eating what my kid’s eating, so what’s the big deal?”
The big deal is that a child with serious peanut allergy doesn’t have to be eating peanuts to have a reaction. When Sophia Falk was 11, she traveled with her Santa Monica-based soccer team to Japan to play in a sister-city tournament. Between games, some of her teammates were eating peanuts. Falk and her family have known about her peanut allergy since she was 2 years old, so Falk doesn’t eat peanuts. “But they were playing cards,” says her mom, Rebecca Haggerty, “so either she got something on her hands from playing cards, or it might have been on the goalie gloves.”
Haggerty had traveled to Japan with her daughter and was there at the tournament when Falk came out of the next game with an allergic reaction. “I said, ‘OK, now how’s your breathing? On a scale of one to 10, if 10 is I can’t breathe, where is it?’” says Haggerty. “And she said, ‘It’s a seven.’ I gave her the Benadryl and we waited for maybe 45 seconds, and I said, ‘Where is it now?’ and she said, ‘Now it’s an eight.’” Falk used her EpiPen and she and Haggerty went, with a translator, to the hospital.
Breathing Room
There are social ramifications to peanut allergy as well. Lila Darnault started kindergarten eating lunch alone in the principal’s office, which she says made her feel “left out and sad.” After much prompting, the school created a nut-free table in
the cafeteria – which was still far from ideal. “I’d be only able to pick one friend to sit with me, and I had to make sure they did not have any nuts in their lunch or snack,” says Lila. When she was in first grade, Lila’s teacher ate Reese’s peanut butter cups and peanut butter Girl Scout cookies in the classroom and called her “dramatic” when she complained.
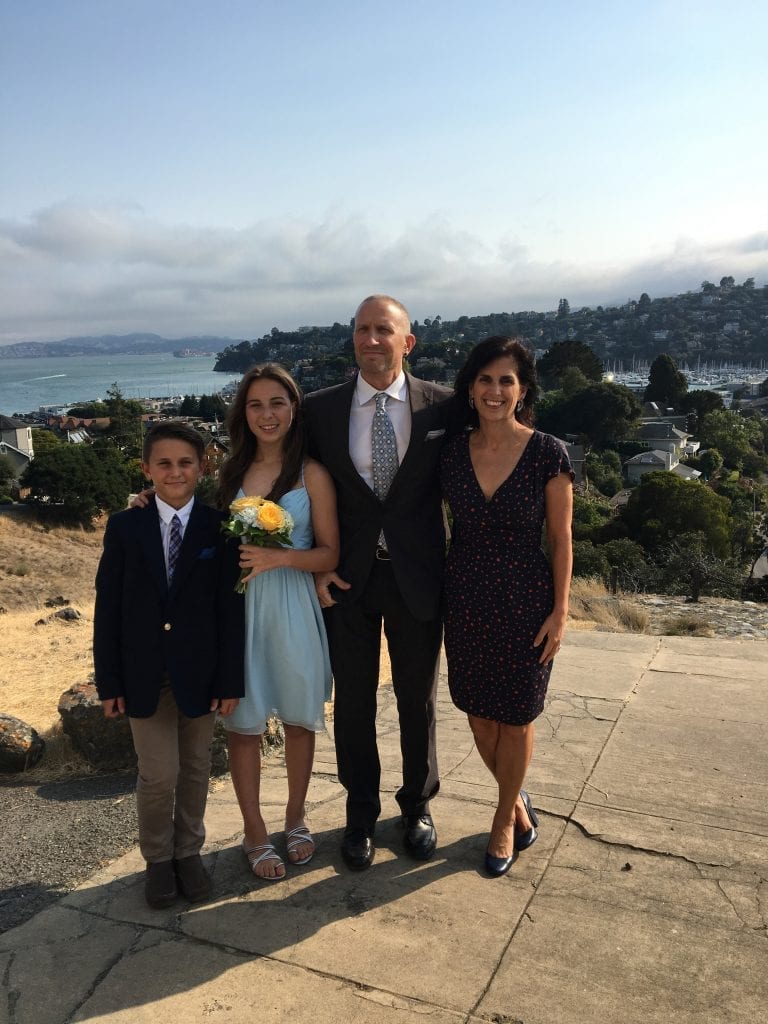
Sophia Falk and her family – mom Rebecca Haggerty, dad Brian Falk and brother Ben Falk – spend much less time thinking about her peanut allergy now that she has been desensitized and can tolerate at least one peanut without trouble. Before therapy, Sophia was hospitalized after allergic reactions to just trace amounts. PHOTO COURTESY REBECCA HAGGERTY
Darnault, who eventually moved Lila to another school, says she wishes there was a term she could use instead of “allergy” to describe Lila’s condition, because people just don’t take it seriously.
When Falk, who is now 15, was in middle school, she was hospitalized after being exposed to a classmate’s PB&J. “We went and talked to the principal,” says Haggerty. “And the principal, who I have a lot of respect for, said, ‘I cannot make this a peanut-free school. That’s not a realistic expectation.’ I get that.” Still, these families do have to rely on others to help keep their kids safe. And OIT could provide an extra safety cushion and lots of peace of mind – especially as kids get older.
Haggerty says she has always tried not to let her anxiety about her daughter’s peanut allergy affect Falk, who is maintaining her newfound peanut tolerance by eating one peanut M&M each day (right before she brushes her teeth, because, like most who are allergic, she is revolted by the taste of peanut). “It’s changed a lot for me,” says Haggerty. “It’s very difficult to watch your kid have a reaction and struggle for breath. That’s a very scary thing to witness. To feel like now I can see her eating a peanut without having a reaction is such a relief.”
Kachru is adamant that OIT cannot cure anyone’s peanut allergy. “You’re giving them a safety zone,” she explains. “We’ve decreased their reaction by tricking the immune system.” But that is enough to transform what would have been an emergency trip to the ER into a minor inconvenience. One of Kachru’s patients, who had completed the trial and was on a maintenance dose of AR101, had this happen at a local restaurant. “She thought she was eating hazelnut ice cream, and it turned out it was peanut ice cream,” says Kachru. Her mouth started to itch, her mom gave her Benadryl, “and that was it.” No EpiPen, no ambulance.
Meanwhile, clinical trials of AR101 are still ongoing at UCLA and other research centers. Some kids weren’t able to tolerate straight OIT, and researchers are trying to use various allergy medications to help make that possible. They are also investigating a peanut immunotherapy patch, which would help patients avoid GI symptoms by introducing peanut allergen through the skin.
UCLA has also opened a clinical OIT program outside the trial, allowing patients who didn’t qualify for the clinical trial but who would benefit from access to the therapy. Thus far, they’ve worked with around 100 patients, with good results.
Still, it could be a while before AR101, which isn’t yet FDA approved, becomes widely available. And the drug isn’t for everyone. “It can feel really scary when you have avoided a food, you have looked at that food like a loaded gun, and the answer to your problem is to give your child that food that you have viewed as poison for so long,” Darnault says. “It felt kind of crazy to have that in my refrigerator.”
But for those it can help, it can make a dramatic difference. “When you can have 100, 200, 300 milligrams of peanut powder and not react, you’re talking about the difference of having a few hives versus a really serious anaphylactic life-threatening event,” says Darnault .The amount of breathing room that I feel like we have because of this is such a game changer.”
Christina Elston is Editor of L.A. Parent.


























